draw all stereoisomers of 1 bromo 4 chlorocyclohexane
Now lets draw the chair structures. Which of the following are optically active.
Solved Chapter 4 Problem 66p Solution Student S Study Guide And Solutions Manual For Organic Chemistry 8th Edition Chegg Com
1-Bromo-3-chlorocyclohexane C6H10BrCl CID 22760233 - structure chemical names physical and chemical properties classification patents literature biological.

. Draw all possible stereoisomers for each of the following. The single bond is active Show the appropriate stereochemistry by choosing the dashed or wedged. Draw all possible stereoisomers for each of the following.
2 1 2 stereoisomers as it has one chiral center k. Write formulas for all of the isomers of each of the following. Draw the molecules on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template toolbars.
Draw all the stereoisomers of 1-bromo-4-chlorocyclohexane. Here n 1 and 21 2 so there are two possible stereoisomers. Now put a Br at C-1 and a Cl at C-4.
The structure with a wedge and a dash is trans-1-bromo-4-chlorocyclobutane. Answer to Solved Draw all stereoisomers of. Usebold and hashed wedges to show the stereochemistryall i know is this is one of them.
Diastereoisomers-are not mirror images but all still connected in same way. 2 -bromo- 4 -methylpentane c. See the answer See the answer See the answer done loading.
On the other put a wedge at C-1 and a dash at C4 trans. 2 2 4 stereoisomers as it has two chiral centers. Draw all the stereoisomers of 1-bromo-4-chlorocyclohexane.
Draw all possible stereoisomers for each of the following. 1 -bromo- 2 -chlorocyclohexane b. 1-Bromo-4-chlorocyclohexane Posted one month ago.
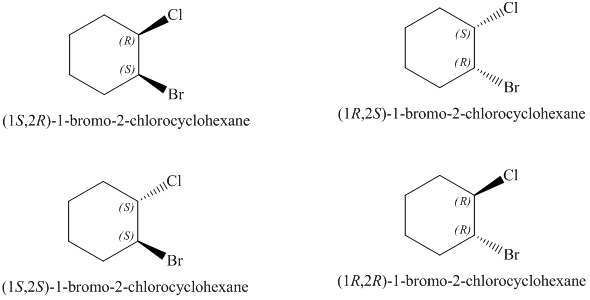
Draw all stereoisomers of 1-bromo-2-chlorocyclohexane Draw all stereoisomers of 1-bromo-2-chlorocyclohexane. This problem has been solved. Show the appropriate stereochemistry by choosing the dashed or wedged buttons and then clicking a bond on the canvas.
The structure with two wedges is cis-1-bromo-4-chlorocyclobutane. State if no stereoisomers are. By definition of cis and trans isomerism the substituent groups when oriented in the same direction constitute the cis isomer and when oriented in the opposite direction constitute the trans isomer.
Draw the bond-line structure for 13-dibromopentane. The two stereoisomers of 4 -bromo-2-pentene i. A 1-Bromo-2-chlorocyclohexane b 1-Bromo-3-chlorocyclohexane c.
Draw the molecules on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template toolbars. Draw all the stereoisomers for each. Calculate the number of possible stereoisomers.
Chemistry questions and answers. Draw all stereoisomers of 1-bromo-4-chlorocyclohexane. Designate pairs of enantiomers and achiral compounds where they exist.
1-bromo 4-chlorocyclohexane has no asymmetric centers therefore it only has CisTrans isomers. Draw the stereoisomers of 1-bromo-3-chlorocyclohexane. The only chiral centre is C-3.
Isomerism is a property of different compounds which exhibit the same molecular number but has different structures and. Draw all possible stereoisomers for each of the following compounds. Draw all stereoisomers of 1-bromo-4-chlorocyclohexane.
Draw two cyclohexane rings. These are the structures of cis and trans isomers for 1-ethyl-3-methylcyclobutane. 1-3Draw three pairs of parallel lines as shown 4-6.
The maximum number of stereoisomers is 2n where n is the number of chiral centres. Draw all the stereoisomers for each of the following compounds. Identify the chiral centres.
Indicate those compounds for which no stereoisomers are possible. Draw all the stereoisomers for each 1 answer below 1-bromo-2-chlorocyclohexane 2-bromo-4-methylpentane. 2 -bromo- 4 -chloropentane e.
Draw equatorial bonds parallel to ring bonds in bold. State if no stereoisomers are possible. Draw all stereoisomers of 1-bromo-4-chlorocyclohexane.
Draw all the stereoisomers for each of the following. Draw all stereoisomers of 1-bromo-4-chlorocyclohexane. 1-bromo-4-chlorocyclohexane exists in two stereoisomers that are cs-isomer and trans-isomer.
Indicate those compounds for which no stereoisomers are possible. Show transcribed image text. Draw all possible stereoisomers for each of the following compounds.
This for organic chemistry college level. Draw all stereoisomers of 1-bromo-2-chlorocyclohexane. The Cl atom will occupy the bonds at C-4 and toggle between axial Cis and equatorial Trans.
The single bond is active by default. 1- chloro- 3 -me 0144. On one put wedges at C-1 and C-4 cis.
Which of the following are optically active. -the more asymmetric centers a carbon has the more stereoisomers it can have-2n of asymmetric centers. 4 -bromo- 2 -pentene h.